Abbott Mine Near Clear Lake, CA (1949)
A Source of Mercury is Abandoned Mines
The Mercury Problem: Global Considerations
Mercury is a basic element forming a significant portion of the earth's crust. The amount present today is identical to that present at the time the earth was formed, but the distribution of mercury has been drastically changed as a result of continued offgassing from the earth and increasingly wide industrial use and subsequent release of fumes from burning coal and oil, and the disposal of mercury-containing products. When mercury ore, or mercury-containing coal, is dug out of the earth vaporization and oxidation occur, creating elemental mercury (Hg0), ionic mercury (Hg++) and particles containing inorganic mercury (Hgpart). In the course of time the mercury compounds become a component of the atmosphere, and begin global distribution. Mercury is unique in that
- it is the only heavy metal that is a liquid at room temperature,
- it is the heaviest liquid in existence,
- it readily vaporizes into the atmosphere,
- it can form stable covalent bonds with carbon,
- most of its compounds are toxic, depending primarily upon their solubility in water and lipids and
- it has no known physiological benefit to living organisms.
In earlier geologic times mercury, as a component of highly insoluble mercury ore (cinnabar), was buried along with other minerals and only released into the atmosphere during earth movements such as volcanoes and similar thermal situations. The natural background levels of mercury have been greatly enhanced in recent times by the release of thousands of tons of mercury and its compounds into the environment as a result of anthropogenic activities such as mining, combustion of contaminated fossil fuels, and disposal of mercury-containing wastes. Consequently we are facing dramatic increases in the levels of mercury spread over the surface of the planet - in the atmosphere, in the oceans, over the land and eventually in living organisms. The industrial revolution has exacerbated a massive transformation from underground (essentially inert) deposits of cinnabar, coal and crude oil to widely dispersed mercury vapor with its capacity for transformation into ionic mercury. In the presence of bacteria in soil and sediment methylation can occur transforming the ionic inorganic mercury into organic mercury potentially increasing its eventual uptake and accumulation in living organisms.
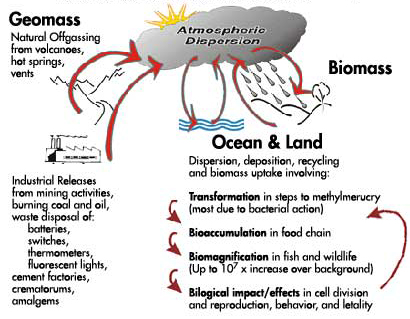
Figure 1 is a simplified version of the mercury biogeochemical cycle. This cycle consists of three phases - terrestrial, oceanic and atmospheric. The major pathways into the atmosphere from natural offgassing by earth movements and volcanoes are depicted on the left along with the varied emissions from industrial activities and disposal of products in which mercury is a component. Inorganic mercury compounds become widely dispersed in the atmosphere and are subjected to repeated deposition and resuspension both over the ocean and over land. In the process of this cycling some of the mercury (Hg0) is oxidized in the presence of ozone, oxygen and moisture to form ionic mercury (Hg++) which is highly water soluble and readily incorporates into rain drops. This ionic mercury (Hg++) is carried by rainwater into soil and sediment containing bacteria, algae, fungi and other organisms. These organisms react with mercury, converting some of it back to its gaseous elemental state for re-release into the atmosphere. Depending upon the conditions and the organisms present in soil and sediment the inorganic mercury can be converted or methylated into organic mercury. The organic mercury (methylmercury MeHg+, and dimethylmercury DMeHg) has the capacity to penetrate through cell membranes and react with essential proteins, amino acids and nucleic acids within the cells. In this form it is highly toxic and capable of being incorporated into the aquatic organisms that are part of the food chain of higher animals. These methylmercury compounds with their capacity to bioaccumulate and biomagnify can compromise the health of higher organisms. This inadvertent exposure to elemental and organic mercury in the environment poses a serious threat to humans as well as wildlife.
Importance of Bacteriological Processes
Bacteria have a uniquely important functional role in the mercury geobiochemical cycle Prior to 1960 it was generally assumed that discharges of elemental mercury remained relatively inert in the environment. More recent work has demonstrated that inorganic mercury can be methylated by organisms present in the soil and sediment of rivers and lakes. Their most significant contribution is the conversion of inorganic mercury to methylmercury by the insertion of a covalent bond between the carbon and mercury atoms. This reaction enables the mercury to penetrate cell membranes more rapidly and accumulate within the cells by complexing with essential proteins, enzymes, and nucleic acids. These reactions demonstrate that all mercury compounds represent a potential threat to living organisms, since in the presence of bacteria, any compound containing mercury can be transformed to the highly poisonous organic sub-stances which are readily incorporated into living tissue.
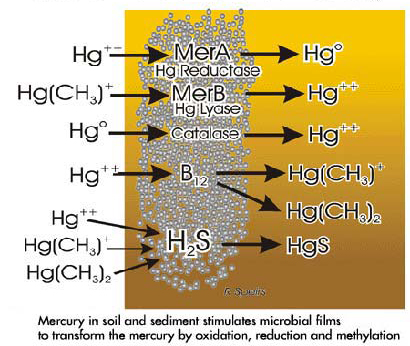
At first glance it is difficult to see what advantages the bacteria gain from methylating the mercury atom. A possible explanation is provided by examining the enzymatic reactions which result in the removal of mercury from within the bacteria and from the immediate vicinity. During the billion or so years bacteria have existed on earth, they have been subjected to continued exposure to mercury in the deep oceans as well as in soil and sediment. Over time they have evolved a capacity to produce enzymes capable of modifying the mercury before and after it has penetrated into the bacterial cells. Masses of bacteria in the form of colonies or films abound in soil and sediment, especially in areas where organic material is plentiful. The composition of the bacteria and the structure of their colonies vary greatly depending upon environmental conditions. In bacterial films the initial exposure to toxic mercury compounds can induce cell death in most of the organisms. However, some organisms can resist the poisoning by induction of specific enzymes which alter the nature and solubility of the mercury, enabling the bacteria to withstand the toxic action. These specific enzymes are coded by DNA found in small organelles called plasmids which can be transferred from one bacteria to another and even from one species of bacteria to another. Following repeated exposure to the toxic mercury compound all the bacteria will eventually become resistant due to the presence of these specific induced enzymes. A wide variety of chemical changes such as oxidation, reduction and/or methylation or transformation of mercury compounds can be produced by these enzymes. In addition hydrogen sulfide, which can react with mercury compounds, can be released (especially during anoxic conditions), forming mercuric sulfide (metacinnabar). Some of these reactions are shown in the figure to the right.
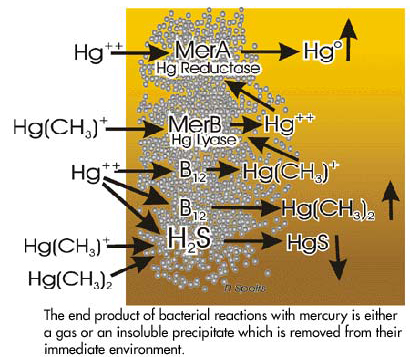
The significant aspect of these enzymatic reactions is that the sum total of effects result in the elimination of mercury, not only from the bacterial cell itself but also from the immediate environment surrounding the bacteria. This is accomplished in the presence of an enzyme called mercury reductase that reduces ionic mercury to elemental mercury which is insoluble in water and readily passes towards the water surface and into the atmosphere and away from the bacteria. This enzyme is coded by a gene in the plasmid called MerA which in the presence of mercury can be induced to form the reductase enzyme. Other enzymes such as an isotype of vitamin B12 (methylcobalamin) can also be genetically induced by the presence of ionic mercury. This enzyme methylates ionic mercury, forming dimethylmercury and monomethylmercury. As indicated earlier the dimethylmercury is highly insoluble in water and forms a gas which passes towards the water surface where it decomposes to monomethyl-mercury and methane. Methylmercury can be produced and/or destroyed by microbial processes. Persisting monomethylmercury within the bacterial films will activate the MerB gene to form mercury lyase which demethylates the mercury. This can then be acted upon by mercury reductase to form elemental mercury which passes to the water surface. In addition under anoxic conditions the presence of hydrogen sulfide will result in the precipitation of mercuric sulfide (metacinnabar) which is highly insoluble and precipitates onto the sediment. The end result of all these enzymatic reactions is the formation of either gaseous or precipitated compounds which remove the mercury from the immediate vicinity of the bacterial film. Thus these organisms have, over time, evolved mechanisms which permit their continued existence, even in the presence of high levels of poisonous mercury compounds. The formation of dimethylmercury may be just one of the means of removing the mercury atoms from the environment which surrounds the bacteria. Some of the reactions are summarized in the figure to the right.
California Aspects
Mercury was a key element in the California Gold Rush due to its role in separating precious metals such as gold and silver from contaminants. This method of processing gold and silver has been an important component of human civilization for thousands of years and was used by Romans to purify gold using procedures that are still in use in some parts of the world to this day. In California individual prospectors could start out with a small leather bag of mercury, pan the streams for small nuggets or flecks of gold, separate the gold from the gravel by amalgamating it with mercury, then vaporizing the mercury and pocketing the gold. This provided a very quick and dirty method for an individual prospector to harvest his treasure.
The extensive panning activity along California's streams soon exhausted the readily available gold and led to the development of hydraulic mining about 1862. Hard rock mining and dredging was also initiated during this period. A very significant step in all of these procedures involved the use of large quantities of mercury to dissolve the precious metal and then harvest the gold by retorting the amalgam.
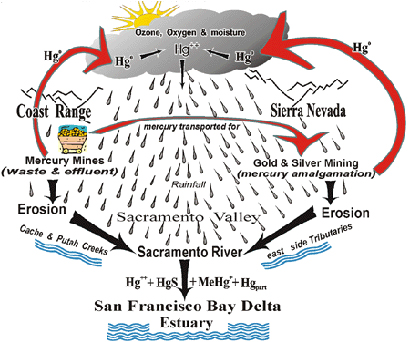
Beginning in 1850 the Coast Range on the west side of the Sacramento Valley provided most of the mercury used in the Gold Rush. Around 300 mines produced approximately 140,000 tons of mercury that supported the California Gold Rush and gold mining activities in Alaska and the whole Pacific Rim. Most of it was transported eastward in 76 pound flasks to the Sierra Nevada and surrounding areas. Due to the inefficient extraction and condensation procedures used in procuring the mercury, and its later use in the amalgamation and separation of the gold and silver, mercury vapor was released into the atmosphere. This resulted in a buildup in the whole Sacramento watershed area. It has remained a major pollution problem for more than a hundred years and will likely continue long into the future. A modified geobiochemical mercury cycle as it applies to the Sacramento Watershed Area is shown to the right.
Mercury is a heavy metal that is unique in that it slowly vaporizes when exposed to the atmosphere and its gaseous elemental form is not water soluble. It becomes water soluble and incorporated into rain drops after being oxidized into ionic mercury (Hg++) in the atmosphere in the presence of ozone and oxygen. The polluted water droplets return the mercury back to earth where it is deposited on soil, some of which is carried by erosion into the waterways. A great deal of the ionic mercury becomes complexed with organic and inorganic particles present in the water. It can also be acted upon by microorganisms to form a variety of organic and inorganic compounds that are eroded into nearby streams and transported into the estuaries of the San Francisco Bay-Delta region. Long after the mercury mining has ceased and gold and silver processing has diminished, mercury and its compounds persist. A permanent legacy of these mercury releases remains not only locally but in snow capped glaciers, peat bogs and sediments located hundreds and even thousands of miles away.
Cache Creek is a tributary of the Sacramento River whose watershed area includes a number of large abandoned mercury mines and a gold mining and processing facility. One of these abandoned mercury mines (the Sulfur Bank Mercury Mine), currently an EPA Superfund site, is still releasing mercury-containing leachate into nearby Clear Lake. This and other mines sites contribute to the contamination of Cache Creek which has been estimated to account for about 50% of the mercury carried into the Bay Delta region each year. During the 1995 high water period it was estimated that approximately 1,000 Kg of mercury, mostly in particulate form was carried downstream into the Delta and Bay areas. (Foe, et al 1997)
Mercury Pollution Considerations
- The Industrial Revolution, which generated widespread contamination of terrestrial and aquatic environments by mercury and its compounds, has greatly enhanced the level of atmospheric mercury.
- The mining of mercury and its subsequent release into the atmosphere, the burning of fossil fuels, cremation off-gasses and disposal of batteries, fluorescent bulbs and other mercury-containing products into landfills have provided a continuing release of mercury which has been transported into the atmosphere and over the entire surface of the earth.
- As it circulates in the atmosphere the mercury is oxidized and settles back to earth in rainfall. When mixed with soil and sediment some of it is transformed to elemental mercury and recycles back into the atmosphere. In addition plants appear to act as a buffer, absorbing mercury when atmospheric levels are high and releasing it when atmospheric levels are lower.
- Mercury and its compounds readily attach to particulate material in soil and sediment. In the presence of living organisms (especially sulfate-reducing bacteria) ionic mercury can transform into monomethylmercury and dimethylmercury. When exposed to sunlight the (di)methylmercury is photodegraded to monomethylmercury, usually near the surface of water, and methane gas is released. Methylmercury is stable and readily penetrates through cell membranes and complexes with essential cellular components.. In humans, methylmercury can be absorbed through the skin or intestinal tract as well as the lungs. It is a cumulative poison which can be highly toxic and/or lethal.
- Mercury and its compounds are cumulative toxins with no known beneficial physiological function in bacteria, plants or animals. The degree of toxicity depends upon the solubility and the capacity for absorption into the organism.
- Once inside the body mercury and its compounds become widely dispersed in blood and tissues and have the capacity to cross the blood-brain barrier and accumulate in the brain and nervous system. It can cross the placenta into the fetus and since it can also become a component of mothers' milk it poses a particular hazard to the fetus and nursing offspring.
- The fetus and infant are much more sensitive (5 - 10x) to mercury toxicity than adults but the symptoms are often delayed or hidden for long periods after exposure.
- In fish, mercury accumulates predominantly in the liver and muscle tissues, primarily in the monomethyl form, which is why it posses a potential human toxicity problem following consumption of fish fillets.
- In humans the most commonly recognized site of mercury damage is the brain. The effects are generally irreversible, resulting in local cell death and, in extreme cases, blindness, coma and death. However less than 10% of the total body burden of mercury is found in the brain and the remainder is distributed in all cells of the body.
- Methylmercury is an extremely effective agent for chromosome breakage and inactivation of the mitotic spindle. It appears to attach to sulfhydryl groups on enzymes and it inactivates the microfilaments involved in cell migration. It is a thousand times more potent than cochicine, the agent usually used in the laboratory to halt mitosis. It can also combine and inactivate DNA and RNA in cells.
- Additional investigation based on cell processes such as mitosis, cell movement and capacity to engulf foreign material is urgently needed to supplement current tests based on mercury's effects on the nervous system.
More Information
Delta Tributaries Mercury Council (DTMC)
The DTMC is a stakeholder group that formed in 1998 under the Sacramento River Watershed Program to provide a forum for the presentation and discussion of mercury issues. The mission of the DTMC is to bring together scientists, regulators, resource managers, water purveyors and interested citizens to develop and collaboratively implement mercury management projects in the Delta watershed and to monitor its effectiveness.
The Mercury Problem: Global Considerations:
Mercury in the Aquatic Environment: A Review of Factors Affecting Methylation
Ullrich SM, et al 2001
Critical Reviews in Environmental Science and Technology 31 (3):241-283
https://www.tandfonline.com/doi/abs/10.1080/20016491089226
Mercury Contamination of Aquatic Ecosystems
By D.P. Krabbenhoft and D.A. Rickert
November 3, 1997
http://water.usgs.gov/wid/FS_216-95/FS_216-95.html
Low-Level Collection Techniques and Species-Specific Analytical Methods for Mercury in Water, Sediment, and Biota
By Mark L. Olson and John F. DeWild
Spring 1999
Download PDF
Importance of Bacteriological Processes:
Microbial Transformation of Mercury Species and their Importance in the Biogeochemical Cycle of Mercury
By Franco Baldi
Metal Ions in Biological Systems, Vol.34: Mercury and Its Effects on Environment and Biology, edited by A. Sigel and H. Sigel, 1997
View on Google Books
California Aspects
Winged Mercury and the Golden Calf
Two elements, one economic theory, and a cascading torrent of collateral damage
By Rebecca Solnit
Orion Magazine, Sept./Oct. 2006
http://www.orionmagazine.org/index.php/articles/article/176/
Mercury Contamination in the Yuba and Bear River Watersheds
A Report of the South Yuba River Citizens League
By Fraser Shilling, Ph.D.
http://www.syrcl.org/issues/Merc&Ars/merc0501.htm
Mercury Contamination from Historic Gold Mining in California
By Charles N. Alpers and Michael P. Hunerlach
http://ca.water.usgs.gov/mercury/fs06100.html
Mercury and Methylmercury in Water and Sediment of the Sacramento River Basin, California (a thorough and detailed study)
Joseph Domagalski, USGS
Applied Geochemistry (2001)16:1677-1691
Download PDF
Mercury Effects, Sources, and Control Measures
Alan B Jones & Darrel Slotton
with contributions from
Chris Foe & Joe Domagalski
1996
Download PDF
Interagency Team Studying Mercury Contamination in California Watersheds
edited by Erin Klaesius May 2000
http://biodiversity.ca.gov/newsletter/v7n2/mercury.html
Mercury From Gold Rush Days Found In California Fish
USGS Press Release
September 28, 2000
http://www.usgs.gov/newsroom/article_pf.asp?ID=568
Environmental Mercury in California
By Walter C. Swain, May, 2000
http://ca.water.usgs.gov/mercury/
Mercury Bioaccumulation in Fish in a Region Affected by Historic Gold Mining: The South Yuba River, Deer Creek, and Bear River Watersheds, California, 1999
By Jason T. May, Roger L. Hothem, Charles N. Alpers, and Matthew A. Law
http://ca.water.usgs.gov/archive/reports/ofr00367/
Minings tarnished legacy - an abandoned mercury mine (Winter 2000)
UC Davis Magazine
http://ucdavismagazine.ucdavis.edu/issues/win00/Feature_Mining.html
Inoperative Mercury Mines fingered as a major source of mercury contamination in California water (11/6/2000)
UC Santa Cruz Currents
http://www.ucsc.edu/currents/00-01/11-06/pollution.html
Mercury Pollution Considerations
Elemental Focus on Mercury (Spring 2001)
BEC Environmental News
http://www.becnet.org/nodes/issues/health/en_2001a_spring_9.php
Chemical Fact Sheet: Mercury (1997-98)
Handbook of Chemistry and Physics
http://www.speclab.com/elements/mercury.htm
